Introduction
There are a wide variety of different options and perspectives on how best to process polymetallic nodules, and there is a divergence of thinking on which process is best.
Three different processes are currently proposed, with minor variants on each:
- Combined pyrometallurgy and hydrometallurgy using RKEF
- Reductive acid leach
- Cuprion
This article will quickly outline each of these processes, how they work, and who is working on each
Pyrometallurgy (RKEF) & Hydrometallurgy
- Currently being pursued by TMC via JV with PAMCO
- Combines Rotarry Kiln Calcination with Electric Arc Furnace (RKEF), followed by hydrometallurgy
- Existing RKEF foundries can be used with likely minimal modifications
- Almost zero-waste production
- Expected recovery rates of 75-95%
This proposed process uses a combination of pyrometallurgical and hydrometallurgical steps in order to refine polymetallic nodules.
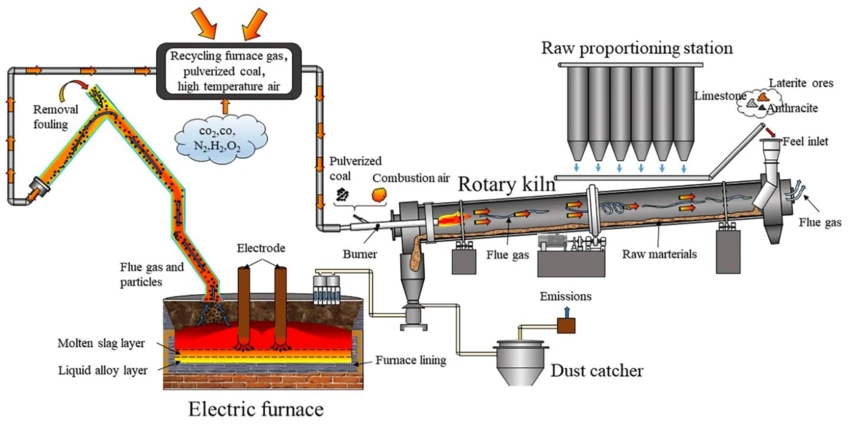
The first pyrometallurgy stage uses an RKEF facility. Nodules are initially mixed with a coal reductant and silica flux (plus any waste residues). These are fed into a Rotary Kiln at around 900°C, where calcination reduces the metal. The calcination discharge is then smelted in an Arc Furnace at around 1350°C to produce a Copper-Nickel-Cobalt alloy and Manganese Silicate. The alloy can be further processed via Pierce-Smith convertors into a matte.

The second hydrometallurgy stage refines the matte into more usable products using a variety of processes including atmospheric/pressure reduction and electrowinning to produce Copper Cathode, Nickel Sulphate and Copper Sulphate.
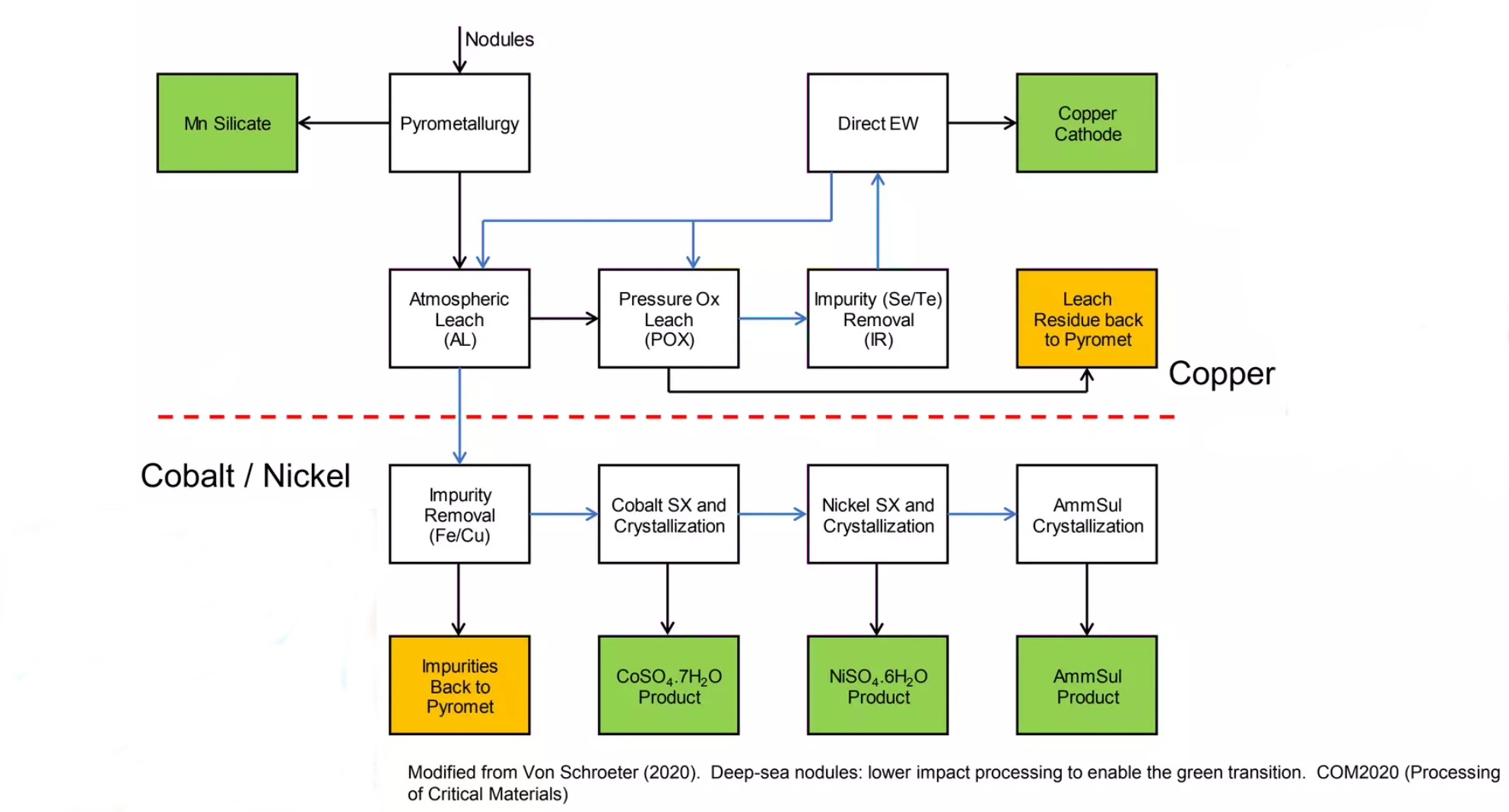
The main advantages of this proposed process are the relatively high recovery rates, the reuse of existing RKEF facilities, and the ability to reprocess waste material back through the RKEF stage, which means that zero solid waste is achievable.
Reductive Acid Leach
- Currently being pursued by GSR / DEME
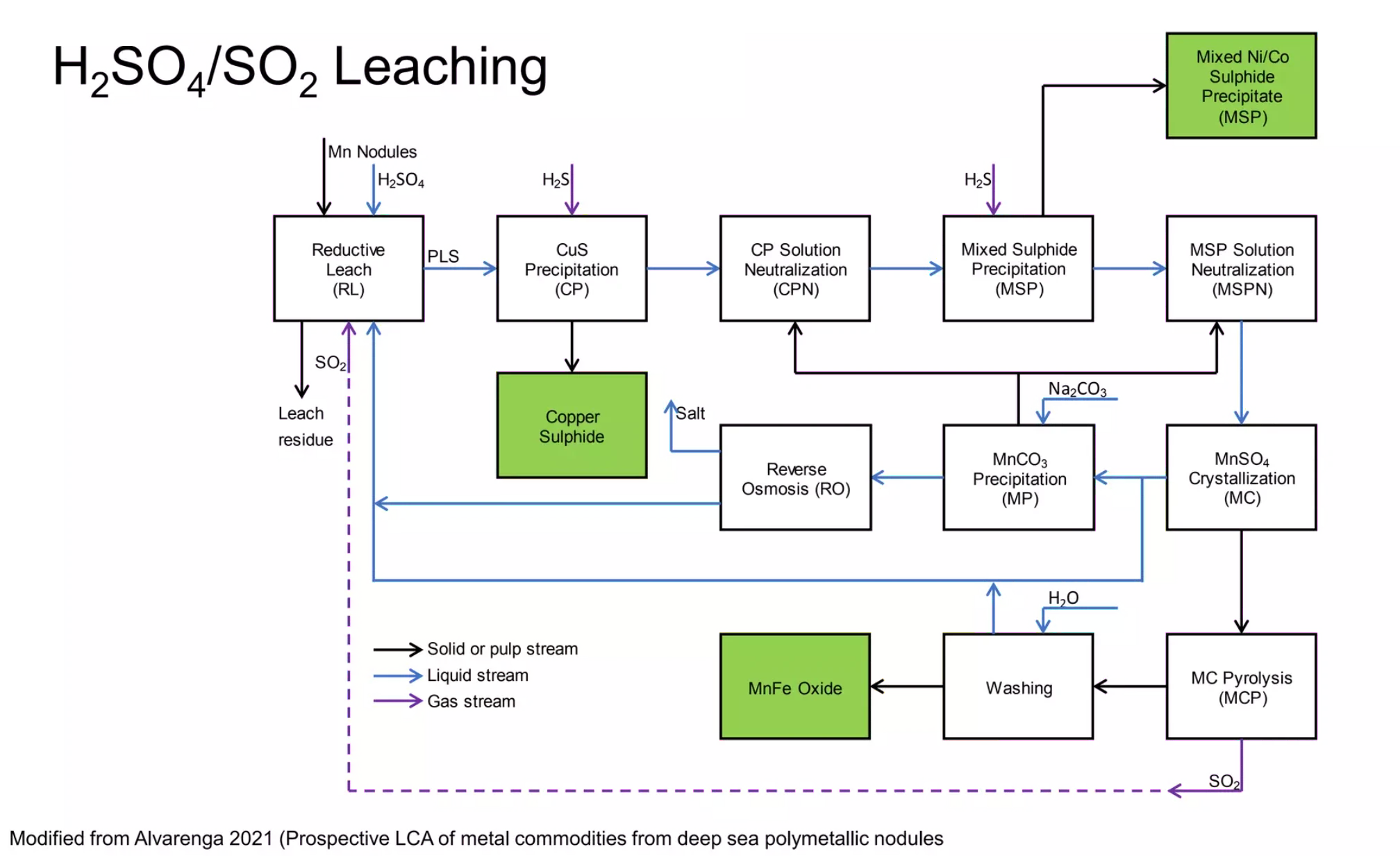
- Single step reductive acid leaching using H₂SO₄ and SO₂
- Very high recovery rates of 98-99% of all metals
GSR has proposed a wholly hydrometallurgical method of processing polymetallic nodules, using a single step acid leach. This uses liquid H₂SO₄ and gaseous SO₂ to precipitate out Copper Sulphide, mixed Nickel / Cobalt Sulphide precipitate, and a mixed Manganese / Iron Sulphate product. Testing has been preliminary, and further development (including building an operating pilot plant) is required.
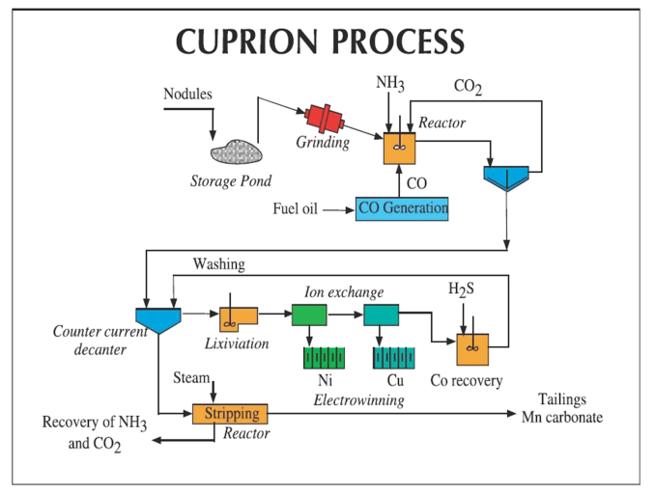
Cuprion Process
The Cuprion Processes uses the Cuprous Ion (Cu+) to destroy the Manganese structure of the nodules, allowing the leaching of the Copper, Cobalt and Nickel through base metal leaching using (NH₄)CO₃.
The process starts by grinding the nodules, in order to create a fine powder and larger surface area. The Cuprous Ion is derived from the Copper content in the nodules themselves, using an Ammonium Carbonate lixiviant, and regenerated using CO gas. This generates the amine Cu(NH₃)₄²⁺, which reacts with the Managanese Dioxide structure of the nodules, destroying it.